Are you seeking for 'how to write balanced ionic equations'? You will find the answers here.
Stairs To Balance Geographic region EquationsWrite the ultimate ionic equation for the unbalanced chemical reaction. ...Separate the ultimate ionic equation into the two half-reactions. ...For one of the half-reactions, balance wheel the atoms demur for O and H. ...Repeat this with the opposite half-reaction.Add H 2 O to balance wheel the O atoms. ...Balance charge. ...Add the two half-reactions together. ...Double-check your work! ...Occupation: Chemical science Expert
Table of contents
- How to write balanced ionic equations in 2021
- Net ionic equation solver
- How to write an ionic equation
- Ionic formula calculator
- Net ionic equation vs complete ionic equation
- How to balance ions
- Net ionic equation examples with answers
- Balanced ionic equation calculator
How to write balanced ionic equations in 2021
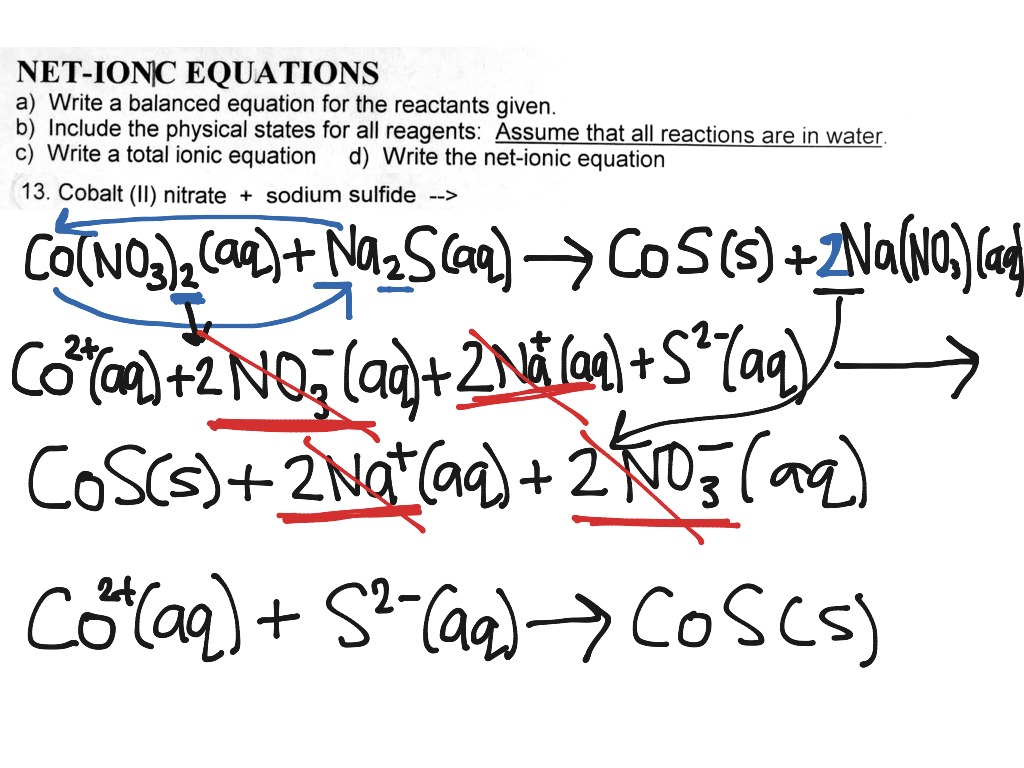 This picture representes how to write balanced ionic equations.
This picture representes how to write balanced ionic equations.
Net ionic equation solver
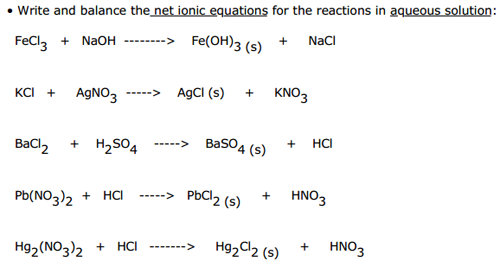 This picture demonstrates Net ionic equation solver.
This picture demonstrates Net ionic equation solver.
How to write an ionic equation
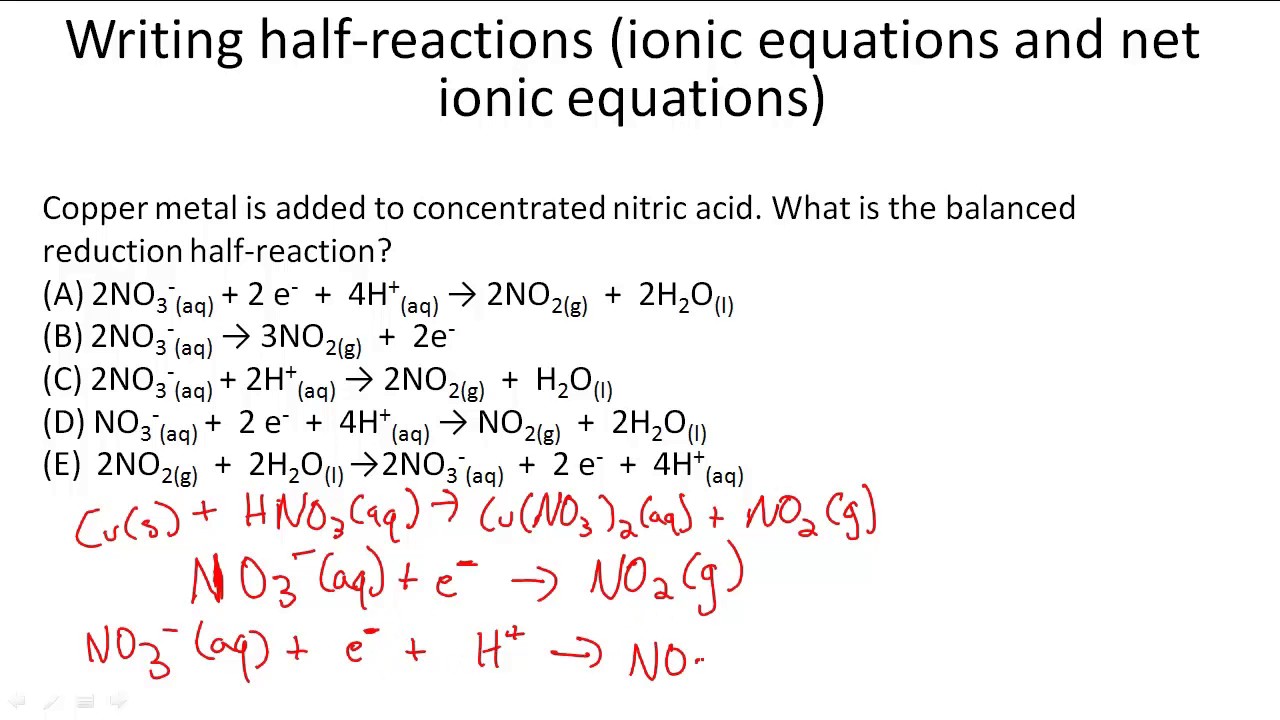 This picture representes How to write an ionic equation.
This picture representes How to write an ionic equation.
Ionic formula calculator
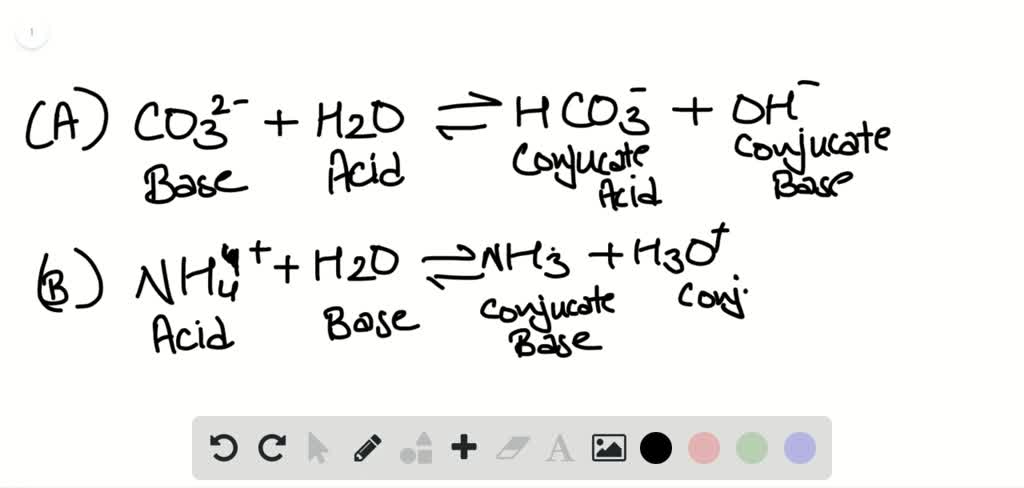 This picture shows Ionic formula calculator.
This picture shows Ionic formula calculator.
Net ionic equation vs complete ionic equation
 This picture illustrates Net ionic equation vs complete ionic equation.
This picture illustrates Net ionic equation vs complete ionic equation.
How to balance ions
 This image illustrates How to balance ions.
This image illustrates How to balance ions.
Net ionic equation examples with answers
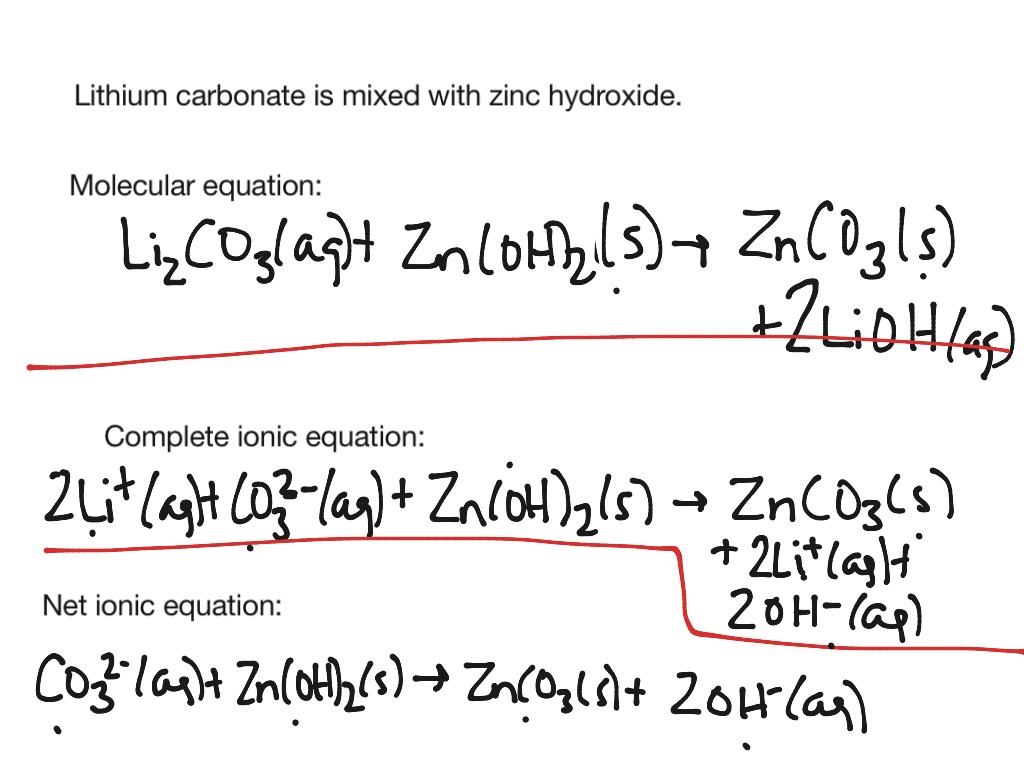 This image demonstrates Net ionic equation examples with answers.
This image demonstrates Net ionic equation examples with answers.
Balanced ionic equation calculator
 This picture shows Balanced ionic equation calculator.
This picture shows Balanced ionic equation calculator.
When do you use a balanced ionic equation?
A balanced ionic equation shows the reacting ions in a chemical reaction. These equations can be used to represent what happens in precipitation reactions. A half equation is used to represent what happens when atoms or ions gain or lose electrons. In half equations: These are half equations for some reactions where positive ions gain electrons:
How are ionic equations similar to chemical equations?
Writing ionic equation is extremely similar to writing chemical equations. Recall that ionic compounds that dissolved in water will dissociate completely into ions (have charge). In an ionic equation: Number of atoms of each elements must be balanced. Total charges carried by the ions must be balanced (e.g. +3 on left must have +3 on right as well)
How to write net ionic equation with spectator ions?
Rewrite the action without any of the canceled species. Spectator ions do not participate in the reaction, but they are present. Finishing the example, there are 6Cl - spectator ions on each side that can be canceled out. The final net ionic equation is 2Cr (s) + 3Ni 2+ (aq) --> 2Cr 3+ (aq) + 3Ni (s).
How to write an ionic equation for neutralisation?
Ionic Equations for Neutralisation STEP 1: Write the chemical equation HCl (aq) + NaOH (aq) ⟶ NaCl (aq) + H 2 O (l) STEP 2: Rewrite by separating the soluble ionic compounds into their dissociated ions H + (aq) + Cl – (aq) + Na (aq) +... STEP 3: Cancel out common ions, which are the spectator ions H ...
Last Update: Oct 2021