Are you searching for 'literature review table of studies'? Here you can find questions and answers on the topic.
Table of contents
- Literature review table of studies in 2021
- Literature review table template word
- Literature review table sample
- Literature review table apa format
- Literature review table pdf
- Literature review table template excel
- Literature review summary table example
- Literature review table apa
Literature review table of studies in 2021
 This picture illustrates literature review table of studies.
This picture illustrates literature review table of studies.
Literature review table template word
 This picture illustrates Literature review table template word.
This picture illustrates Literature review table template word.
Literature review table sample
 This picture shows Literature review table sample.
This picture shows Literature review table sample.
Literature review table apa format
 This image representes Literature review table apa format.
This image representes Literature review table apa format.
Literature review table pdf
 This picture shows Literature review table pdf.
This picture shows Literature review table pdf.
Literature review table template excel
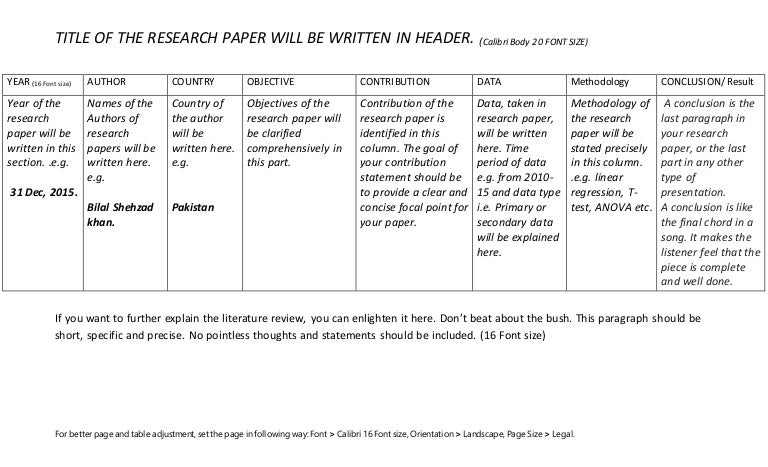 This image representes Literature review table template excel.
This image representes Literature review table template excel.
Literature review summary table example
 This image shows Literature review summary table example.
This image shows Literature review summary table example.
Literature review table apa
 This picture shows Literature review table apa.
This picture shows Literature review table apa.
How are systematic reviews included in literature review tables?
Systematic reviews and metaanalyses are included in Table A-1; studies and articles are included in Table A-2. Number of Studies, Inclusion Criteria, and Databases Searched Ruth-Sahd, L. A. 2003. Reflective practice: A critical analysis of data-based studies and implications for nursing education. Journal of Nursing Education 42 (11):488-497.
What are the rules for a Chicago style literature review?
Finally, if you are required to write a literature review in Chicago style, here are the key rules to follow: Set page margins to no less than 1 inch. Use double spacing across the entire text, except when it comes to table titles, figure captions, notes, blockquotes, and entries within the bibliography or References.
What kind of studies are included in a literature review?
A wide range of designs were classified as informative, including randomized controlled trials, prospective cohort studies, observational studies, and studies with pre- and post-intervention assessment methodologies. Quantitative and qualitative approaches were included, and inclusion was not limited to studies with positive results.
What are the characteristics of included studies tables?
Participants: setting; relevant details of health status of participants; age; sex; country. Sufficient information should be provided to allow users of the review to determine the applicability of the study to their population, and to allow exploration of differences in participants across studies.
Last Update: Oct 2021